Prolific Machines—a California-based startup harnessing light-sensitive proteins to control cells used in biomanufacturing—has raised $55 million in a series B1 round led the Ki Tua Fund, the corporate venture arm of New Zealand dairy co-op Fonterra Co-operative Group.
The first close of the round, which takes the firm’s cumulative funding to $86.5 million, was supported by Breakthrough Energy Ventures, Mayfield, SOSV, Shorewind Capital, Darco Capital, Conti Ventures, In-Q-Tel (IQT), and several others.
Founded by stem cell biologist Dr. Deniz Kent (CEO), physicist and biomedical scientist Dr. Max Huisman (CTO), and computer scientist and ML engineer Declan Jones (CIO) in 2020, Prolific Machines introduces light-sensitive proteins to cells that are used to biomanufacture everything from vaccines to high-value ingredients used in infant formula.
Rather than controlling cell function by adding expensive growth factors or other molecules to cell culture media, as companies do now, Prolific Machines exposes cells containing light sensitive proteins to light at specific wavelengths, a technique known as optogenetics. This can activate or deactivate certain genes, proteins, or cellular pathways, delivering “unprecedented cellular control,” CEO Dr. Deniz Kent tells AgFunderNews.
“This is the first time light’s ability to control cellular functions is being used outside of research labs to make everyday essentials. It’s incredibly exciting and the start of something very big for lots of different industries.” Dr. Maximilian Hoerner, head of optogenetics, Prolific Machines
Transitioning from using molecules to control cells to using light
For decades, says Kent, “We’ve been using molecules—whether chemicals or proteins—to control cells, and they come with a bunch of problems.”
In some cases, the issue is cost, he notes, with some growth factors used in therapeutic proteins (IGF, EGF), stem cell cultivation (FGF, TGF-β), tissue engineering (VEGF, PDGF), vaccine production (GM-CSF, IL-2) and gene therapy (FGF2, HGF) coming with hefty price tags.
But this is not the only problem, claims Kent: “You can’t control where these molecules go, and when they go where they go. We’re just dropping them in bioreactors and hoping for the best. Another issue is sterility [any component added to a bioreactor increases the risk of contamination], plus there are reproducibility problems because some of these molecules are biological in nature and so they are not consistent.
“You can buy FGF2 [fibroblast growth factor 2, a widely-used regulator of cell development, differentiation, regeneration, senescence, proliferation, and migration] from the same supplier with the same catalog number, but it won’t necessarily be the same as the FGF2 that you bought a month ago or a year ago.”
Light is cheap, inherently sterile, reproducible, and can be controlled spatially and temporally
He adds: “Every one of those problems is solved with light, the cheapest possible input into biology, plus it’s easy to control both spatially and temporally, that is, you can control where it goes and when it goes where it goes, which allows you to get higher yields. Controlling on the space axis also allows you to create patented products that other people won’t be able to make using molecules that are moving around randomly.”
Light is also inherently sterile, eliminating contamination risk [introducing molecules to bioreactors always carries the risk of contamination], and is inherently reproducible, tackling the inconsistency problem, he adds. “If you have 450 nanometer wavelength light today it is the same as it will be tomorrow and a thousand years from now.”
Another advantage of using light rather than molecules added to cell culture media to direct cellular activities is that it can potentially reduce downstream processing and purification costs post-harvest, as there are fewer media components you need to ensure do not end up in your final product, he says.
One the regulatory side, meanwhile, “there’s a number of advantages of using light because it doesn’t carry the same potential risks of chemicals or recombinant proteins,” claims Kent. “We’ve spoken to the FDA about this on several occasions, and they were very happy about the fact that we have a solution to eliminate the needs for these other inputs.”
“Breakthrough innovations like Prolific Machine’s biomanufacturing platform don’t come along often — particularly innovations that have the potential to unlock profound impact in both human and environmental health, across multiple industries.” Komal Mistry-Mehta, fund director, Ki Tua Fund
How it works
According to Kent: “We take light sensitive proteins [of which there are thousands] and we genetically attach them to different targets inside the cell. You can do that by making permanent genetic changes or via transient genetic changes [which would impact whether the cell would be considered a ‘GMO’ for regulatory purposes].”
Targets inside the cell could be “anything from a receptor on the surface of cells, an enzyme, a transcriptional activator, something that turns one type of cell into another, or something that tunes cell metabolism to make more ATP [which provides energy for biochemical processes] and less lactic acid [which can limit cell densities] per molecule of glucose. And you can toggle the activity of that target using light.
“What we’ve invented is a way for machines to control some cellular biology specifically for the first time.”
As the mammalian cells that Prolific Machines is focusing in are not naturally light sensitive, the only things that respond to light in the bioreactors are the targets that it has tagged with light sensitive proteins, enabling great precision, he says.
“We have hardware that illuminates the cells. And then we have software that is taking all of the spectral data that we’re generating, and then learning [using machine learning] what’s the right pattern of light to apply. We don’t just leave the light on all the time; there are different patterns of light to do different things.”
For example, he says, with traditional approaches to biomanufacturing, we often ask cells to do multiple things at the same time, such as grow and produce protein.
“With light it is easy to separate out those phases,” which might be more efficient, he says. “You can use one color of light to accelerate the growth with no [protein] production, and then switch to a different color to end growth and only focus on production. So you can reduce the amount of time it takes to get to maximum cell density and improve titers [the amount of an expressed target molecule relative to the volume of liquid].”
Initial focus on mammalian cells
To date, Prolific Machines has focused on mammalian cells, although there is no reason to believe the tech wouldn’t work with microbial cells used in precision fermentation, for example, says Kent, who says the company will be announcing commercial partnerships in due course.
“This is in part because there’s a lot more investor appetite to do mammalian applications than there is to do microbial applications, because you can make a lot more money, so we’ve initially focused on things like antibodies and cytokines, vaccines, viral vectors for gene therapy, and tissue engineering.”
Asked if anyone else is currently deploying optogenetics in biomanufacturing, Kent says: “As far as we know, we’re the first company to use light in this way to aid biomanufacturing. But there are other companies that are using light in other contexts. For example I’m aware of a company that is using light to reverse blindness by genetically engineering the cells of your eyes to become light sensitive. There is also a lot of activity in the neuroscience field.”
Prolific Machines has 14 patent families, “11 of which are owned by us and then three of which are owned by universities (Stanford, Berkeley, Johns Hopkins),” he says. “But we have exclusive licenses to them.”
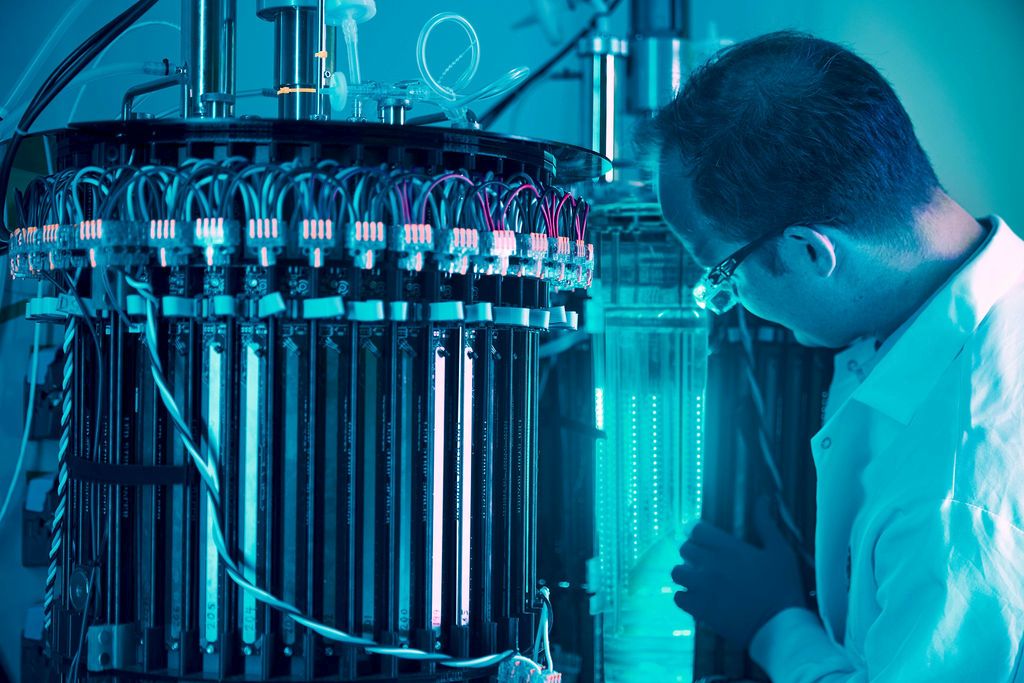
Implementing the technology: ‘Think of us as the NVIDIA for biology’
Prolific Machines’ technology has been “intentionally designed in a way that makes it very easy for people to use,” says Kent. “For example, the genetic constructs do not need to be in our cell lines; we’ve designed them in a way that they can slot into any one’s cell lines, because some people have spent years optimizing their cell lines.
“Think of us as the ‘NVIDIA for biology,’ where we can elevate our partners’ existing cell lines and product approaches, providing a critical infrastructure layer for biology.”
Similarly, specialist bioreactors are not required, he adds: “All they need to do is buy our hardware that slots into their existing bioreactor infrastructure. The software is embedded in our hardware.”
To expose the cells to light, many bioreactors already have glass portholes through which light can be directed, he says. And as cells are being agitated, “you don’t need to illuminate 100% of the bioreactor to ensure that 100% of the cells are illuminated,” he explains.
“We’re also building internal illumination systems, so you could have lights on rods that go into the bioreactor, lights on the impeller [which stirs or agitates the contents of the bioreactor to ensure all cells access the nutrients], or you can add neutrally buoyant balls [containing LEDs] floating around in there.”

Cultivated meat… a ‘far more elegant approach’
Cultivated meat is one use case for Prolific Machines’ technology given the potential to eliminate the use of pricey cell culture media components, says Kent.
While some firms in this space are now using CRIPSR gene editing techniques to edit cells such that they don’t need to use certain growth factors, there are drawbacks to this approach, he claims.
“If you’re using CRISPR to get your cells to express growth factors themselves, your cells are constantly pumping out a lot of growth factor, and you can’t tune that, whereas with lights, you can very precisely tune how much growth factor activation you want at different times.
“The other problem with [the CRISPR approach] is that if your cells are constantly producing their own growth factors, more than they need, you’re putting a big metabolic burden on the cells, which slows them down. There is a sweet spot, and it shifts over time depending on what’s going on in the bioreactor. So using light is just a far more elegant approach.”
Further reading:





